Aliphatic compounds are an important class of compounds in terms of the amount and complexity of optically active substances. The structure of the aliphatic compound is non-rigid and is subject to change. The conversion energy barrier between different conformations is small, and the proportion of these conformations in the equilibrium system is susceptible to external conditions. Theoretically, the free rotation of each CC single bond produces an infinite number of conformations. It is therefore the most difficult class of compounds to analyze and study the relationship between optical rotation and structure.
Although each CC single bond in this type of compound produces an infinite number of conformations, there are only three energy-lowest crossovers and three highest energy overlapping conformations, all of which are between the crossover and the overlap. Conformation. In the conformational equilibrium system of each CC bond at normal temperature, the total percentage content of the three cross-conformations is about 99% or more. This percentage actually consists of a conformation with an angle of intersection close to 60 degrees, since the energy of these conformations differs slightly from the cross-conformation energy of 60 degrees, and the relative average position of the six covalent bonds in these intermediate conformations, the angle of intersection They are all 60 degrees. At room temperature, there is little overlapping conformation in the conformational equilibrium system. Therefore, in general, when analyzing the relationship between structure and optical rotation, only three cross-conformations and their percentages are considered, and the overlapping conformation is not considered. Only a single specific compound in a solvent with a small polarity or in a gas phase may have a near-overlapping conformation. This occurs when a highly electronegative atom replaces a hydrogen atom on C1 in propane. Because of the large electronegativity of the substituted atoms, the inductive effect causes them to carry excess negative charges. For the same reason, the hydrogen atoms on their neighboring atoms carry a partial positive charge. Fluorine, oxygen, chlorine atoms and hydrogen atoms in the ortho C have strong electrostatic attraction. Thus, the close to the overlapping conformation is rather stable. In polar solvents such as water and alcohol, this overlapping conformation is eliminated by solvation. In determining the most stable conformation, it is also important to note that the two substituents are usually the most stable conformation in the para-crossing. If hydrogen bonds or some other gravitational force can be formed between the two substituents, the ortho-crossing will also become the most stable conformation (as shown below).
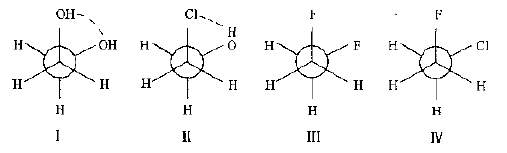
Several compounds with a stable conformation
It can be seen that in the analysis of the relationship between structure and optical rotation, special attention should be paid to conformational analysis. When determining a stable conformation, pay attention to whether hydrogen bonds and forces between dipoles can be formed. The role of the solvent cannot be ignored in the conformational analysis.
Van der Waals force and bending keys
In an organic compound molecule, each atom and other atoms that do not bond with it, whether in the same molecule or in different molecules, have both gravitational and repulsive forces. This force is called Van der Waals force. When two atoms are far apart, gravity is dominant. When the distance is close, the repulsion is dominant. When the gravitational and repulsive forces are exactly equal, the distance between them is the sum of the van der Waals radii of two atoms. When the 2 atoms are closer together, a net repulsion will occur. The closer the distance, the greater the net repulsion. For example, methane is the simplest organic compound, with four CH bonds having a bond length of 0.109 nm and six bond angles of 109.28 degrees. The van der Waals force radius of the hydrogen atom is 0.12 nm. The distance between any two hydrogen atoms in methane (0.18 nm) is less than the sum of the van der Waals radii between hydrogen atoms, meaning that there is a repulsive force between any two hydrogen atoms in methane, officially due to the formation of hydrogen atoms. The equal repulsive force between the key tracks, the bond angle between the four CH keys is 109.28 degrees. Only by dealing with this state, the distance between the hydrogen atoms and the bonding orbitals is the farthest, the repulsive force is the smallest, and the internal energy of the molecules is the lowest. If two hydrogen atoms are replaced by atoms with a larger van der Waals radius, the bond angle must increase because the repulsive force is large. A compound containing a simple chiral carbon atom. Since four carbon atoms or groups are attached to each other on a chiral carbon atom. Their van der Waals radii vary and their repulsive forces vary. The four bond angles on the chiral carbon atoms must be curved and the directions of the bends are different. The atom or group to which such a chiral carbon atom and the four bonds around her form a six-and-a-half helix. When the curvature of the four keys is not too large, then the six spirals should be four right-handed spirals, two left-handed spirals, or four left-handed spirals, and two right-handed spirals. The magnitude of the repulsive force of the four atoms or groups attached to a chiral carbon atom is determined primarily by the polarizability of the atoms directly bonded to the chiral carbon atom. The greater the polarizability, the smaller the valence electrons of the outermost layer of the atom are bound by the nucleus. The further the outer valence electron is from the nucleus, the greater the repulsion between this atom and the outer electrons of other substituted atoms. The greater the polarity of the valence electrons in an atom, the more susceptible it is to the electromagnetic field of plane-polarized light, that is, the greater contribution to the optical rotation. Therefore, the repulsive force of the atom directly bonded to the chiral atom can be polarized and optically contributed, and the order of the three sizes is uniform. Because the atomic species in most organic compounds are limited by several non-metal atoms. In general, the larger the atomic radius, the larger the covalent radius. The larger the covalent radius, the smaller the bond of the 2 atom-pair valence electrons, and the valence electrons are easily polarized by the external electric field, and the refractive index is also larger, so the contribution to the optical rotation is greater.
Metal Disposable Fiber Optic Laryngoscope
Disposable Laryngoscope Set,Neonatal Laryngoscope Blade Sizes,Fiber Optic Laryngoscope Blades,Laryngoscope Blades And Handles
Jiangsu Yongle Medical Technology Co., Ltd. , https://www.jsylmedical.com