1. Introduction
It’s estimated that approximately 20% of all small-molecule pharmaceuticals are halogenated. Carbon-halogen bonds play a crucial role in enhancing various properties of compounds, such as thermal and oxidative stability, as well as improving their ability to cross biological membranes. In the field of drug discovery, natural products (NPs) serve as biologically significant scaffolds, and halogenation can further optimize their characteristics. A previous study showed the potential of chemical halogenation in plant extracts to yield bioactive compounds. Consequently, a strategy for the generic halogenation of plant extracts was developed to create libraries of unique halogenated NPs, particularly for identifying novel antifungal agents. Initially, the halogenation reaction was tested on a series of NP standards from different chemical classes. Once successful, this method was extended to various plant extracts using bromine (Br) and iodine (I) [3].
Â
2. Oxidative Halogenation of Natural Products
This research aimed to utilize an eco-friendly halogenation process that employs sodium halides in an aqueous hydrogen peroxide solution and apply it to a variety of pure NPs and crude extracts to assess whether this approach could enhance their antifungal activities.
Halogenation Procedure
- Chlorination: 6 equivalents NaCl – 1g standard – 6 equivalents H2O2 – 10mL acetic acid – diluted to 25mL with water
- Bromination: 6 equivalents NaBr – 1g standard – 6 equivalents H2O2 – 10mL acetic acid – diluted to 25mL with water
- Iodination: 3 equivalents NaI – 1g standard – 6 equivalents H2O2 – diluted to 25mL with water
- Reaction Time: 24 hours
- Conducted at room temperature
- Performed under magnetic stirring
- Follows green chemistry principles
Â
Chemical Reaction
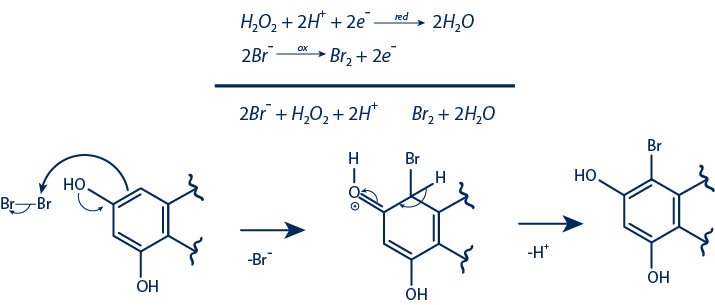
Semi-Synthetic Compounds Obtained from Oxidative Halogenation
Yields ranged from 0.7% to 29%
A series of NP standards with diverse scaffolds were chosen for halogenation. Each compound was subjected to the reaction with 50 mg of material. The purification of the halogenated derivatives was carried out using reverse-phase semi-preparative HPLC-UV.
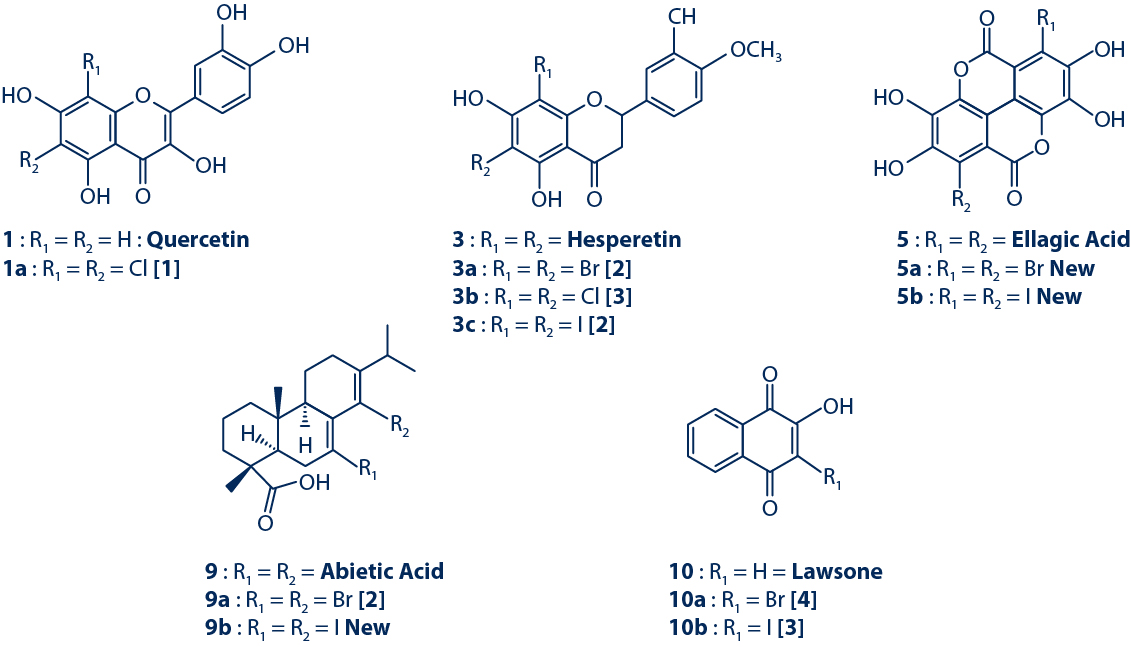
3. Antifungal Assay Against C. albicans
The antifungal activity of the purified halogenated derivatives was assessed against C. albicans using both the bioautography assay (MIQa) and dilution assay (MIC).
C. albicans
Strains:
CAF 21
DSY 2621
Antifungal Bioautography Assay
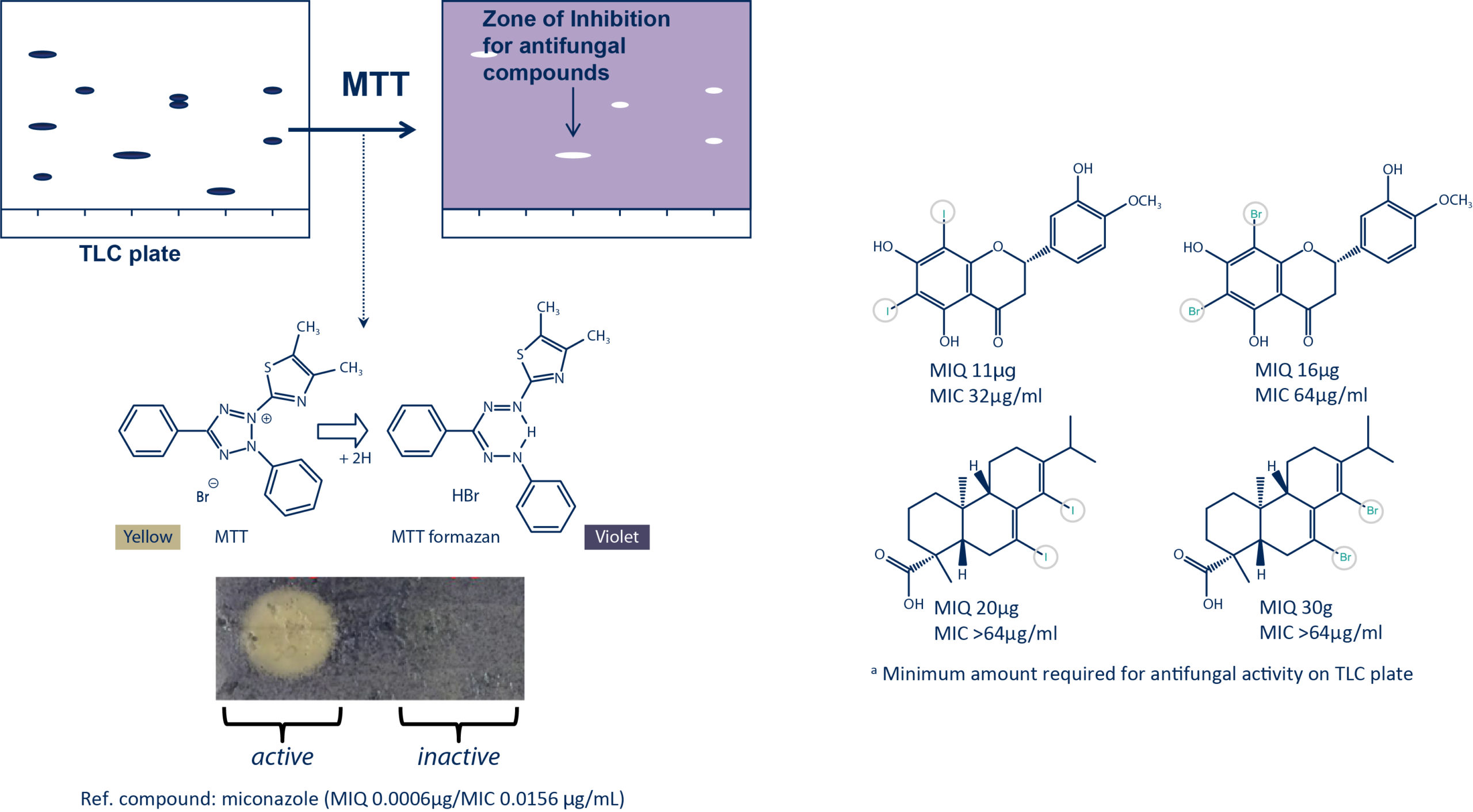
Â
4. Selection of Crude Extract Rich in Flavonoids
Citrus Species: 8.5% World Fruits Production
 40-60% of oranges are used for fruit juices 50% of the pericarp constitutes the total weight |
After validating the halogenation reaction with the series of standards, the methodology was applied to a model crude plant extract (Citrus sinensis pericarp), which is rich in flavonoids and readily available at large scales. |
Citrus sinensis Pericarp Constituents
| Components | wt% on the basis of dry weight |  |
| Ash (%) | 2.56 | |
| Sugar (%) | 9.57 | |
| Fat (%) | 4.00 | |
| Protein (%) | 9.06 | |
| Flavonoid (%) | 4.50 | |
| Pectin (%) | 23.02 | |
| Lignin (%) | 7.52 | |
| Cellulose (%) | 37.08 | |
| Hemicellulose (%) | 11.04 |
Â
5. Bromination of the Citrus Pericarp Methanolic Extract
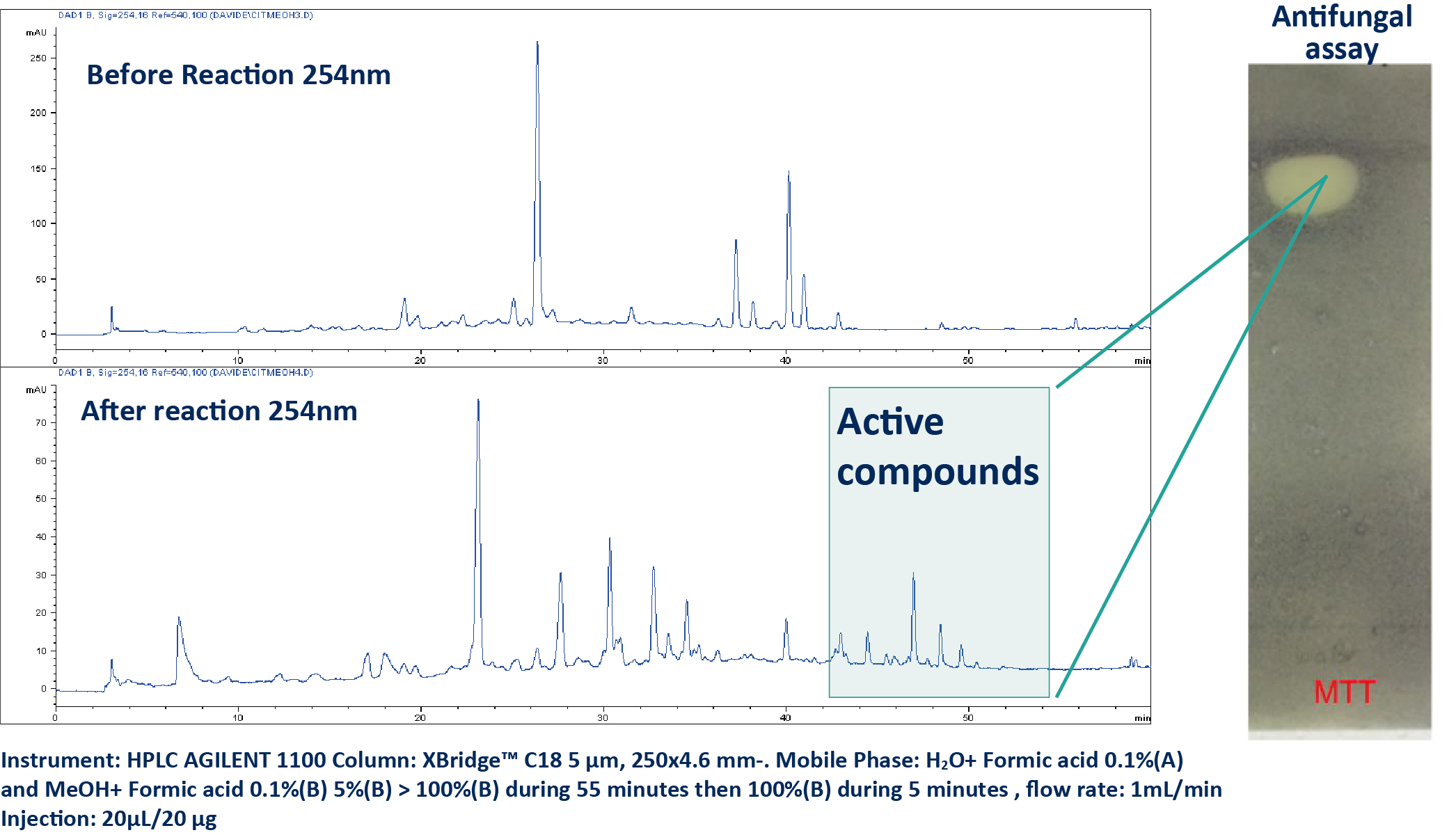
The halogenation of the methanolic extract was initially conducted at the analytical scale using 100 mg of the extract over 24 hours. Afterward, the reaction was halted and monitored via HPLC-UV (A). The crude reaction mixture exhibited strong antifungal activity against C. albicans.
To isolate these active compounds, the reaction was scaled up to 1 g while maintaining the same reaction time (24 hours) (B). The reaction mixture was dried and purified through normal phase flash chromatography (FC). The preparative conditions were initially determined at the analytical scale and then transferred to the preparative scale using the same stationary phase [5]. The fractions obtained were dried and subjected to the antifungal assay, leading to the identification of four active compounds (1-4). The structural elucidation of these compounds was performed using HRMS and NMR.
a. HPLC UV Analysis of the Crude Extract Before and After Reaction
b. Normal Phase Ultra Pressure Flash Chromatography (FC-UV)
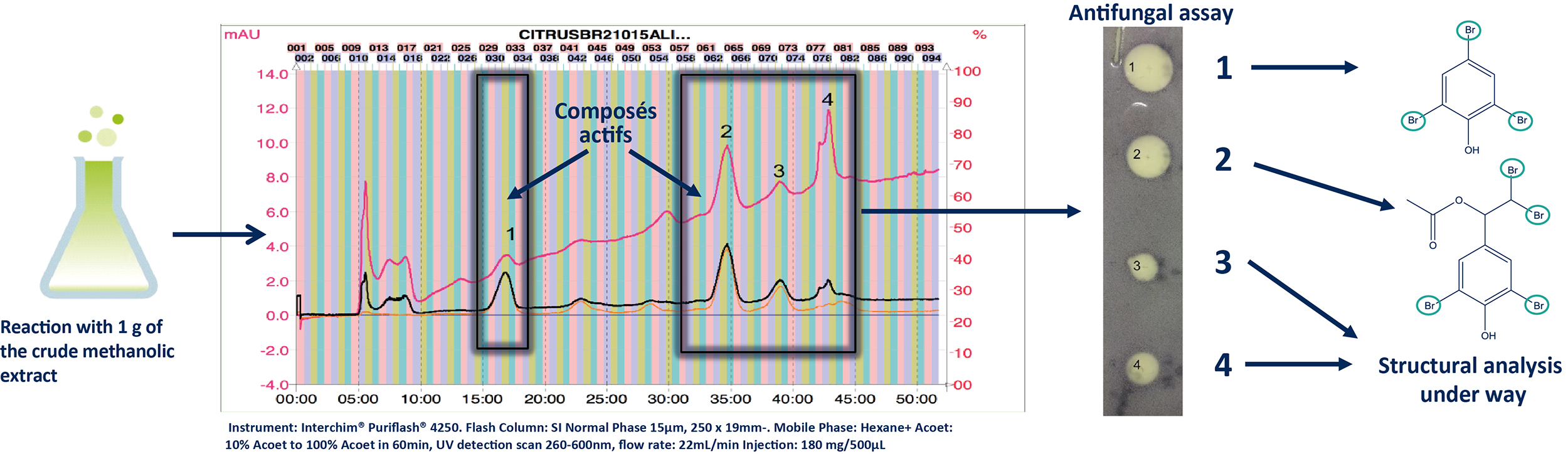
Â
6. Conclusion
The halogenation of a series of NP standards proved effective in producing antifungal compounds against the human fungal pathogen Candida albicans. The reaction was performed under eco-friendly conditions using mild reagents. The methodology was also successfully applied to the halogenation of a complex crude plant extract derived from a waste material (citrus pericarp), resulting in unusual antifungal halogenated derivatives.
These preliminary findings further highlight the potential of chemical halogenation of plant extracts to yield bioactive 'unnatural' natural products of interest. In this context, applying such reactions to crude extracts from waste biomass could hold significant value in producing valuable bioactive NPs.
The next step will involve evaluating the halogenated derivatives against different biological targets and extending this methodology to a broader range of plant extracts with chemically diverse compositions.
Â
7. References
1. Herrera-Rodriguez et al. Chem Today 2011;29:4
2. Mendez et al. ACS Comb Sci. 2011;13:200-204
3. Bernini et al. New J Chemi. 2015;39:2980-2987
4. Favre-Godal et al. Phytochemistry 2014;105:68-78
5. Challal et al. Planta Med. 2015; 81: 1636-1643
Â
Learn More:
 |
|
Gaba Sleep Drink ,Sleep Drink ,Sleep Well Drink ,Melatonin Drink
Guangzhou Green health Pharmaceutical Technology Co.,Ltd , https://www.greenhealthfactory.com