EPC Isolation and Characterization
1. EPCs were obtained by isolating mononuclear cells using Ficoll density-gradient centrifugation of human blood buffy coats.
2. After resuspension in endothelial basal medium (EBM-2) supplemented with EGM-2 containing vascular endothelial growth factor, basic fibroblast growth factor, insulin-like growth factor-1, epidermal growth factor, and 5% FBS, 10 6 mononuclear cells /cm 2 were plated on fibronectin-coated tissue culture flasks.
3. After 4 days of culture, nonadherent cells were discarded by washing with PBS.
4. To confirm the EPC phenotype, adherent cells were incubated with DiI-labeled acLDL for 1 hour and after fixation were incubated with FITC-labeled Ulex europaeus agglutinin I (ulex-lectin) for 1 hour.
5. Cells were visualized with an inverted fluorescent microscope, and adherent cells staining positive for both FITC–ulex-lectin and DiI-acLDL were judged to be EPCs.
6. Staining of nuclei with DAPI verified that nearly all adherent cells (>95%) were acLDL(+)ulex-lectin(+).
7. Cells were subsequently detached and analyzed by flow cytometry to determine the light-scattering properties of the acLDL(+)ulex-lectin(+) cell fraction.
EPC Surface Molecule Analysis
1. Cells were detached with EDTA and labeled for 20 minutes at 4°C at manufacturer-recommended concentrations with fluorescent antibodies: anti-VE-cadherin-PE and anti-E-selectin–FITC as intermediate markers; anti–CD11b-PE ( Mac-1), anti-CD11c-PE, and anti-CD14-APC as monocyte/macrophage markers; anti-CD45–FITC as a panleukocyte marker; anti–AC133-APC and anti–c-kit-PE as stem/progenitor Cell markers; and anti–CD31-PE and anti–CD34-APC, which are not specific to a single cell lineage but have been detected previously on cultured EPCs.
2. Fluorescent isotype–matched antibodies were used as negative controls.
3. Cells were washed, paraformaldehyde-fixed, and analyzed on a FACS-Calibur Instrument with ≥10 000 events stored.
4. Data are presented as mean±SEM percentage of positive cells corresponding to the acLDL(+)ulex-lectin(+) cell gate in ≥3 experiments.
Comparison of Circulating Cells and Cultured EPCs
1. The expression of surface markers on circulating monocytes and cultured EPCs was compared.
2. Buffy-coat leukocytes were labeled on day 0 and cultured EPCs on day 4 with the following antibodies: anti–CD11c-PE as a monocyte/macrophage activation marker, anti–CD34-APC as a stem/progenitor or endothelial marker, anti –CD45–FITC as a panleukocyte marker, and anti–CD163-PE as a marker of monocyte-to-macrophage differentiation.
3. Anti–CD-14-APC was used to define the CD14+ light-scatter gate on circulating leukocytes and cultured EPCs.
4. The cultured cells were labeled as above, the labeling of circulating leukocytes in the buffy coat involved the addition of FACS lysing solution to lyse contaminating erythrocytes in the buffy coat. Data are shown as representative histograms of surface molecule expression on circulating monocytes And cultured EPCs of the same donor in the light-scatter gate corresponding to CD14+ cells.
EPC Proliferation
1. Bromodeoxyuridine (BrdU) was added to EPC flasks 4 days after isolation.
2. Flasks without BrdU served as negative controls.
3. On day 4, cells were detached with EDTA and labeled with an anti-BrdU–FITC antibody and the DNA stain 7-AAD.
4. Cells were analyzed for FITC positivity and cell cycle position with a FACS-Calibur instrument.
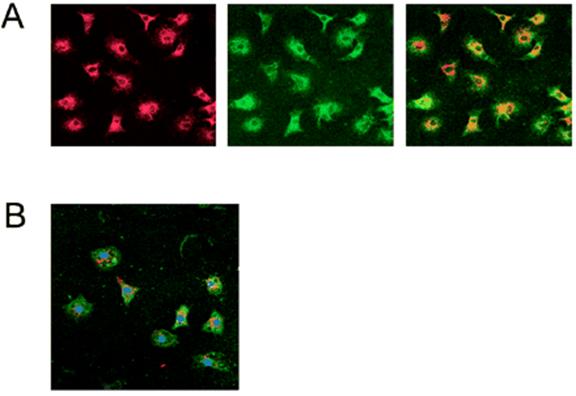
A, Fluorescence microscopy (40× Objective) illustrates that adherent cells were positive for uptake of DiI-labeled acetylated LDL (left) and binding of FITC–ulex-lectin (center). All acLDL(+) cells were also positive for ulex- Lectin binding, as can be seen in overlay (right), and therefore corresponded to current definition of cultured EPCs. B, Confocal microscopy (40× objective) of EPCs with nuclear stain DAPI (purple) demonstrates that all nuclei are found in cells that Are acLDL(+) (red) and binding of ulex-lectin(+) (green), thus illustrating that all adherent cells are acLDL(+)ulex-lectin(+). It was observed that a modest number of loosely adherent smaller Cells did not take up acLDL, but these cells were lost during multiple washing, staining, and fixation steps.
Reference
Kalka C, Masuda H, Takahashi T, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci US A. 2000; 97: 3422–3427.
Vasa M, Fichtlscherer S, Aicher A, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001; 89: E1–E7.
High Quality Safety Goggle,Safety Glasses Goggle,Industrial Safety Goggles,Eye Protection Goggles
GULF STAR MEDICAL MANUFACTURING FZE , https://www.gulfstarmedical.com